FAQ & Spec.
Frequently Asked Questions
WHAT IS WOUNDVIEWER?
How long does it take to measure the parameters of the lesion with woundviewer?
What are the parameters monitored?
what languages are available?
is data protection guaranteed?
do you have a privacy policy?
Technical Specifications
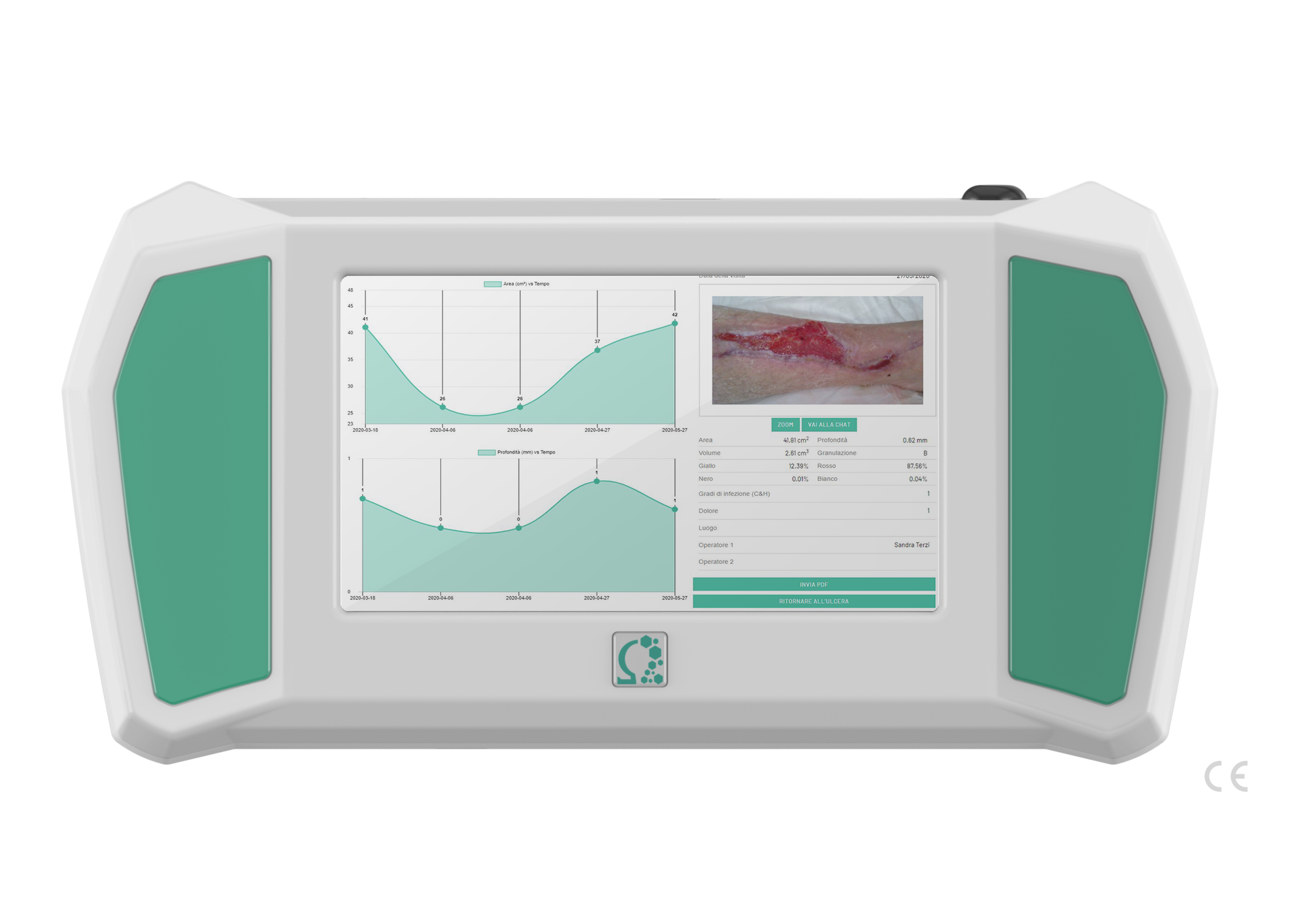
DISPLAY
- LCD interface capacitive
- Touch Screen
- 2 USB Plugs
CAMERA
- 1 x CMOS Camera
- 16 x IR Sensors
- 4 x White LEDs
- Microphone
- Geo-localization
- microSD
COMMUNICATION
- WiFi interface
- 4G interface
- Bluetooth
- Micro – nano SIM
REGULATORY STANDARDS REQUIRED
compliance with the following standards:
[S1] EN ISO 13485 Medical devices – Quality management systems – Requirements for regulatory purposes.
[S2] EN 60601-1 Medical electric equipment Part 1: General requirements for safety.
[S3] EN 60601-1-11 Medical electrical equipment General requirements for basic safety and essential performance — Collateral standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment
[S4] EN 60601-1-2 Medical electric equipment Part 1: General requirements for safety 2- Collateral Standard: Electromagnetic compatibility- Requirements and tests.
[S5] EN 62304 Medical device software – Software life-cycle processes.
[S6] EN ISO 14971 Medical devices – Application of risk management to medical devices.
[S7] 1907/2006 Regulation (EC) of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACh), establishing a European Chemicals Agency
[S8] 2011/65/EU Directive of the European Parliament and of the Council of 8 June 2011 on the restriction of the use of certain hazardous substances (RoHS) in electrical and electronic equipment (recast).
[S9] EN ISO 14971 Medical devices – Application of risk management to medical device
[S10] EN 62366-1 Medical devices. Application of usability engineering to medical devices
Frequently Asked Questions
WHAT IS WOUNDVIEWER?
HOW LONG DOES IT TAKE TO MEASURE THE PARAMETERS OF THE LESION WITH WOUNDVIEWER?
WHAT ARE THE PARAMETERS MONITORED?
WHAT LANGUAGES ARE AVAILABLE?
IS DATA PROTECTION GUARANTEED?
DO YOU HAVE A PRIVACY POLICY?
Technical Specifications

DISPLAY
- LCD interface capacitive
- Touch Screen
- 2 USB Plugs
CAMERA
- 1 x CMOS Camera
- 16 x IR Sensors
- 4 x White LEDs
- Microphone
- Geo-localization
- microSD
COMMUNICATION
- WiFi interface
- 4G interface
- Bluetooth
- Micro – nano SIM
REGULATORY STANDARDS REQUIRED
compliance with the following standards:
[S1] EN ISO 13485 Medical devices – Quality management systems – Requirements for regulatory purposes.
[S2] EN 60601-1 Medical electric equipment Part 1: General requirements for safety.
[S3] EN 60601-1-11 Medical electrical equipment General requirements for basic safety and essential performance — Collateral standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment
[S4] EN 60601-1-2 Medical electric equipment Part 1: General requirements for safety 2- Collateral Standard: Electromagnetic compatibility- Requirements and tests.
[S5] EN 62304 Medical device software – Software life-cycle processes.
[S6] EN ISO 14971 Medical devices – Application of risk management to medical devices.
[S7] 1907/2006 Regulation (EC) of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACh), establishing a European Chemicals Agency
[S8] 2011/65/EU Directive of the European Parliament and of the Council of 8 June 2011 on the restriction of the use of certain hazardous substances (RoHS) in electrical and electronic equipment (recast).
[S9] EN ISO 14971 Medical devices – Application of risk management to medical device
[S10] EN 62366-1 Medical devices. Application of usability engineering to medical devices
Frequently Asked Questions
WHAT WOUNDVIEWER?
HOW LONG DOES IT TAKE TO MEASURE THE PARAMETERS OF THE LESION WITH WOUNDVIEWER?
WHAT ARE THE PARAMETERS MONITORED?
WHAT LANGUAGES ARE AVAILABLE?
IS DATA PROTECTION GUARANTEED?
DO YOU HAVE A PRIVACY POLICY?
Technical Specifications

DISPLAY
- LCD interface capacitive
- Touch Screen
- 2 USB Plugs
CAMERA
- 1 x CMOS Camera
- 16 x IR Sensors
- 4 x White LEDs
- Microphone
- Geo-localization
- microSD
COMMUNICATION
- WiFi interface
- 4G interface
- Bluetooth
- Micro – nano SIM
REGULATORY STANDARDS REQUIRED
compliance with the following standards:
[S1] EN ISO 13485 Medical devices – Quality management systems – Requirements for regulatory purposes.
[S2] EN 60601-1 Medical electric equipment Part 1: General requirements for safety.
[S3] EN 60601-1-11 Medical electrical equipment General requirements for basic safety and essential performance — Collateral standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment
[S4] EN 60601-1-2 Medical electric equipment Part 1: General requirements for safety 2- Collateral Standard: Electromagnetic compatibility- Requirements and tests.
[S5] EN 62304 Medical device software – Software life-cycle processes.
[S6] EN ISO 14971 Medical devices – Application of risk management to medical devices.
[S7] 1907/2006 Regulation (EC) of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACh), establishing a European Chemicals Agency
[S8] 2011/65/EU Directive of the European Parliament and of the Council of 8 June 2011 on the restriction of the use of certain hazardous substances (RoHS) in electrical and electronic equipment (recast).
[S9] EN ISO 14971 Medical devices – Application of risk management to medical device
[S10] EN 62366-1 Medical devices. Application of usability engineering to medical devices